
Op-ed: How the FDA Ignores the Law When Approving New Chemical Additives to Food

The FDA has consistently reviewed individual chemicals without regard to the cumulative effect on chronic disease. LauriPatterson / Getty Images
By Maricel V. Maffini and Thomas G. Neltner
The U.S. Food and Drug Administration’s (FDA) failure on food chemical safety has left consumers at risk of chronic diseases.
The agency is required to review the safety of classes of chemicals rather than individual chemicals. Using the class approach, multiple chemicals adversely affecting the same organ or system (such as the immune, endocrine, or nervous systems) are evaluated together and a safe consumption level is determined for the class. This approach prevents the intentional new or expanded uses of chemical additives that increase chronic disease and, when coupled with a systematic review of prior decisions, results in health risk reduction. Instead, the agency has consistently reviewed individual chemicals without regard to the cumulative effect on chronic disease.
In the last 60 years, innovations in processing, preserving, and packaging have made food more affordable, convenient, and available. To accomplish this transformation, industry, with the FDA’s approval, has brought thousands of chemicals into the food system, resulting in diets increasingly composed of ultra-processed foods without regard for the cumulative effect of these additives and their long-term chronic health consequences.
When Congress passed the Food Additive Amendment in 1958 in response to a rapidly changing food system and rising public and scientific concerns about the potential health risks of new chemical additives, it included a health-protective requirement: the cumulative effect of chemically and pharmacologically related substances in the diet must be taken into account when assessing the safety of new additives. That means, additives with similar toxic effects, either because they look alike or affect similar body functions, must be evaluated together to prevent exposures above an amount that would cause harm.
However, food manufacturers and regulators have neglected to consider this cumulative effect, failing to harness changes in food technology and use advances in scientific knowledge to protect the public from dietary chemical exposures. Medical associations and a group of health, environmental and consumer organizations have jointly challenged the FDA to change its practice of not accounting for the cumulative health effect of chemicals in the diet as required by law.
We Are Sick
A lot of us are affected by chronic health conditions. Diabetes in children and adults; attention, learning and memory disorders; obesity in children and adults; thyroid dysfunction; and the list goes on. Experts call them non-communicable diseases because, unlike pathogens like bacteria and viruses, we do not pass them from one person to another. Global public health experts linked tobacco use, physical inactivity, alcohol abuse, and unhealthy diets to increases in the risk of non-communicable diseases.
Unhealthy diets are usually associated with calorie-dense nutrient-poor foods, often called ultra-processed foods, due to their ingredients resulting from a series of industrial processes, many requiring sophisticated equipment and technology (sweet and savory snacks, reconstituted meats). In addition to industrially produced ingredients (high-fructose corn syrup, protein isolates, hydrogenated oil), such food also contains numerous additives including dyes, flavors, emulsifiers, thickeners, and artificial sweeteners. Further, industrial chemicals used in packaging manufacturing and food processing equipment —such as bisphenol A (BPA), phthalates, PFAS, perchlorate— are also found in these foods.
These intentional uses of chemical additives number in the thousands, and many have been linked to endocrine disruption, neurological and behavioral problems, cancer, and heart and liver disease.
Congress Added Guardrails Against Chronic Health Effects
In the U.S., approximately 10,000 chemicals can be purposely added to food or enter the food supply through processing equipment and packaging, and 60 percent of the calories ingested are from ultra-processed foods. In 1958, Congress gave the FDA authority to regulate chemicals intentionally added to food or to food contact materials, commonly known as food additives, to ensure their use is safe. Safe means the potential toxic health effects of a new additive that becomes part of the diet must be assessed in combination with other substances already present and are expected to have similar health effects. Thus, the cumulative assessment of health effects by a class of related substances prevents the addition of intentional new or expanded uses of chemical additives that would increase chronic disease. Moreover, this approach, together with systematic review of prior safety decisions results in health risk reduction.
FDA Neglected Its Responsibility to Follow the Law
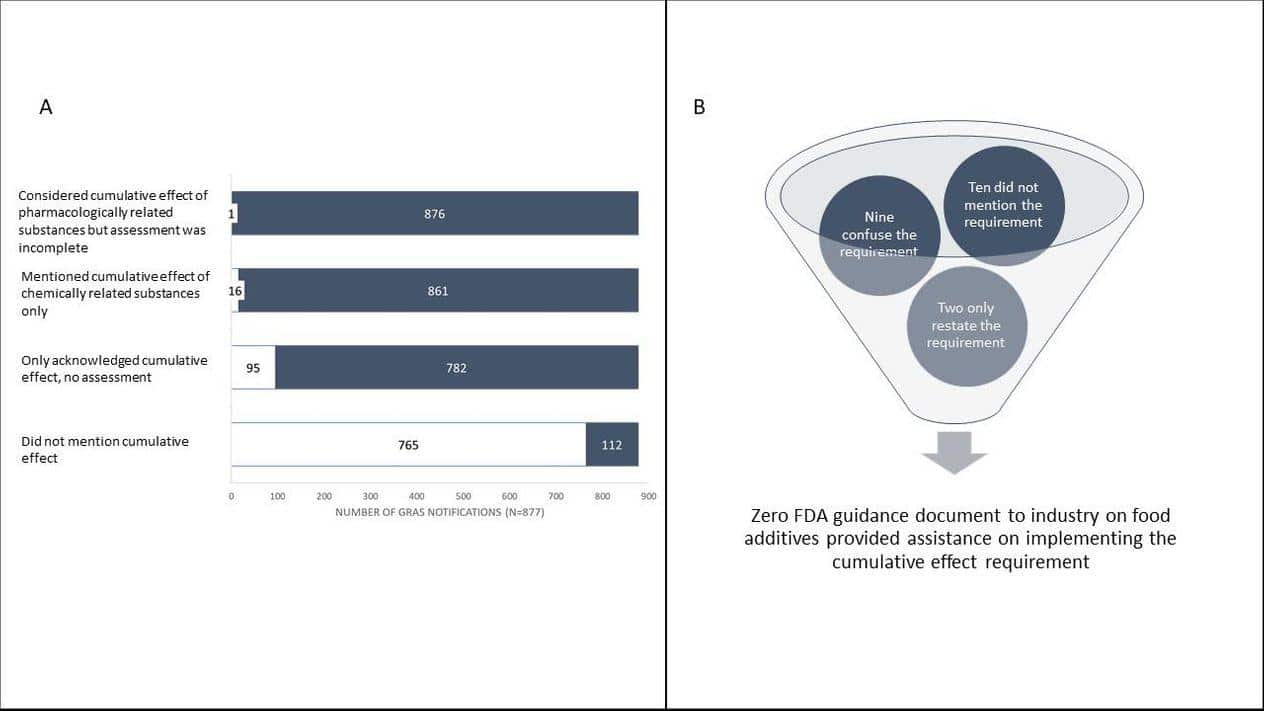
We wanted to investigate whether and how food manufacturers and the FDA had implemented the cumulative effect requirement. To do that, we downloaded and reviewed all 877 safety determinations contained in the Generally Recognized as Safe (GRAS) notifications inventory. These notices were voluntarily submitted by food manufacturers to the FDA between 1997, when GRAS notification program began, and March 24, 2020. We looked at GRAS notices because they are publicly available and FDA rules require that food manufacturers include in the notice an explanation of how they considered the cumulative health effect of a new additive. Unfortunately, our investigation showed that both the FDA and the food manufacturers appeared to have ignored this crucial safety requirement.
We searched the documents for terms “cumulative effect” and “pharmacological” presuming that any analysis of the cumulative effect of chemical or pharmacologically related substances would include those terms. We evaluated every positive finding for context and reviewed the document more closely when warranted. We found that in only one of 877 GRAS notices did a food manufacturer consider the cumulative effect requirement in a meaningful way. Notably, that one notice stopped short of establishing a safe exposure for the class as required by regulation. And we found no evidence that the agency either recognized this single attempt to follow the law or had objected to the omissions in the 876 other notices.
To better understand how these blatant omissions happened, we also reviewed the FDA’s relevant guidance for industry documents to determine if they contain information to help industry understand how to consider the cumulative effect of the substance as required by law and regulations. We used the agency’s online research tool and identified 21 documents related to food chemicals. For each document, we searched for key terms including “cumulative effect”, “chemically related”, “pharmacological effects”, and “pharmacologically related”. We also searched for references to key regulations or statutory provisions directly related to the cumulative effect requirement. We found next to nothing and what information was there was either incomplete or confusing.
Ten documents did not mention the legal requirement and two simply restated it. Four documents created confusion by using terms such as ‘cumulative exposure’ or ‘cumulative intake.’ Five documents provided incomplete and potentially misleading information. For example, excluding the requirement from the definition of safety or paraphrasing the safety requirement in a manner that limited the assessment to a single chemical instead of related substances in the diet.
The Unknown Cost of FDA’s Six Decades of Failure
This is an obvious failure by the FDA and food manufacturers that has significant consequences for public health, particularly for communities already facing significant health and socio-economic disparities and for children, who are uniquely susceptible to dietary exposures to multiple chemicals. It is known that fetal and early life exposures have been associated with long-term diseases or disorders that usually manifest later in life. Development of neurological, immune, reproductive, and endocrine systems have been shown to be particularly susceptible to chemical exposures. For example, several food additives and contaminants in common foods – including nitrates, perchlorate, thiocyanate, BPA, phthalates, potassium bromate, synthetic dyes – all harm the thyroid’s ability to produce a hormone essential to brain development. The common-sense preventative measure to reduce exposures is to treat chemicals in the diet with related health effects as a class – as Congress mandated in 1958.
The healthcare costs of long-lasting health conditions, especially when they arise during childhood, as well as the economic benefits of preventing exposures to substances that disrupt the normal function of the endocrine system have been documented.
How can this be remedied?
Solutions
First, the FDA needs to add definitions of key terms such as “cumulative effect”, “chemically related”, “pharmacologically related” and “pharmacological effect.” This should not be a heavy lift. For instance, the agency’s own Center for Drug Evaluation and Research has already established definitions for pharmacologically related substances and pharmacological effects; food additive regulators could also implement this. We are not implying that additives be regulated as drugs; rather, that the body does not identify whether a chemical that binds to a hormone receptor is a pharmaceutical or a food additive. But it certainly may have a similar biological response with potentially different health consequences depending on the dose, duration of exposure and life-stage of the individual.
Second, the FDA should review the requirement for all forms industry must complete when submitting petitions or notifications to the agency for review of their products’ safety assessment. FDA should provide clear and specific guidance to industry on what it is expected and how to accomplish it. And, of course, the agency needs to ensure compliance with the law.
Medical and scientific societies together with health and environmental organizations have formally submitted a petition to the FDA to revise its food and color additive regulations and associated guidance to ensure compliance with the requirements in law. Safe food is fundamental to protect the health and well-being of all Americans. Putting into action the protections already available in the law and regulations would also restore the confidence in the FDA’s mission to protect the public health by assuring the safety of our nation’s food supply.
Lastly, these efforts should be conducted without delay so we begin to curb the epidemic of chronic diseases that continue to inflict personal and financial pain in so many families and worsen an already strained healthcare system.
Reposted with permission from Environmental Health News.

 233k
233k  41k
41k  Subscribe
Subscribe 