

Kevin Maillefer / Unsplash
By Lynne Peeples
Editor’s note: This story is part of a nine-month investigation of drinking water contamination across the U.S. The series is supported by funding from the Park Foundation and Water Foundation. Read the launch story, “Thirsting for Solutions,” here.
In late September 2020, officials in Wrangell, Alaska, warned residents who were elderly, pregnant or had health problems to avoid drinking the city’s tap water — unless they could filter it on their own.
More than 3,000 miles (4,800 kilometers) away, the people of Scituate, Massachusetts, received a letter that same month cautioning about the same group of contaminants in their drinking water.
At issue wasn’t any of the well-known and widely feared water infiltrators such as E. coli or per- and polyfluoroalkyl substances (PFAS). The culprit chemicals tainting taps from Cocoa, Florida, to the Finger Lakes of New York to a correctional facility in Only, Tennessee, are, in fact, less recognized yet more ubiquitous: disinfection by-products.
“Take a glass of water. You may or may not have pesticides, pharmaceuticals, PFAS and lead in it. Usually not,” says Susan Richardson, a professor of biochemistry at the University of South Carolina in Columbia. “But there’s always something that is in your drinking water, and that’s disinfection by-products.”
Aptly named, the chemicals form in water when disinfectants that are widely used to kill pathogens in municipal drinking water facilities react with organic compounds. These compounds may be present in the water as a result of natural processes such as the decay of leaves and animal matter, as well as human activities that may release solvents, pharmaceuticals, pesticides and industrial chemicals. Exposure to disinfection by-products through drinking, bathing or swimming has been linked to potential increased risks of low birthweight babies, birth defects, miscarriages and cancer.
“Disinfection is hugely important. We’ve got to kill those pathogens,” says Richardson. “We had millions of people dying from waterborne illnesses before we started disinfecting water in the 1800s.”

Cholera and typhoid fever were once deadly and pervasive threats. Still today, when concentrations of disinfectants fall too low, drinking water can become a breeding ground for dangerous pathogens such as Legionella, E. coli, even cholera.
“It’s a trade-off between inactivating pathogens that are going to make people sick today versus the long-term, low-level risk of chemicals in the water,” says Christy Remucal, an associate professor of civil and environmental engineering at the University of Wisconsin–Madison.
Striking a balance may be even more challenging today as waters become increasingly compromised due to population growth, wastewater intrusion, energy exploration, climate change — and now the Covid-19 pandemic, according to Richardson.
During the pandemic, many places have increased use of chlorine for disinfection in indoor and outdoor settings and during wastewater treatment, resulting in the potential for higher levels of disinfection by-products. Authors of a study published in October warn that this “upsurge and overuse of chlorine-based disinfectants” may pose a threat to human health “by impacting water quality.”
Concentrations of harmful chemicals have also likely increased in buildings left vacant during Covid-19 shutdowns. The longer that water sits in pipes, explains Richardson, the longer it has to react with disinfectants and form more by-products.
Still, Gregory Korshin, a professor of civil and environmental engineering at the University of Washington in Seattle, encourages perspective on the issue of disinfection by-products. The answer, he and others say, is not to stop disinfecting water, nor is it for everyone to buy bottled water.
“There is a dark side of disinfection,” adds Korshin. “But this doesn’t compromise the notion that drinking water in the U.S. is safe.”
Unintended Consequences
Chemists first discovered disinfection by-products in treated drinking water in the 1970s. The trihalomethanes they found, they determined, had resulted from the reaction of chlorine with natural organic matter. Since then, scientists have identified more than 700 additional disinfection by-products. “And those only represent a portion. We still don’t know half of them,” says Richardson, whose lab has identified hundreds of disinfection by-products.
Identification of disinfection by-products is incredibly difficult, she explains, because these chemicals are not simply flowing down a river from an industrial site or running off a farm. “They didn’t exist before,” she adds. “It’s a complete unknown — there’s no preconceived idea of what these chemicals look like.”
Another research team recently discovered more previously unidentified disinfection by-products. As they described in a January 2020 study, potentially carcinogenic chemicals are formed through the interaction of chlorine and not only organic matter in the environment but also manmade materials that include phenols such as bisphenol A (BPA) and other plasticizers, as well as sunscreen agents and antimicrobials.
“These phenol compounds are incredibly widespread because of their properties,” says Carsten Prasse, a coauthor on the study and an assistant professor of environmental health and environmental engineering at Johns Hopkins University. He highlights their use in both plastic pipes and plastic bottles, which frequently carry drinking water.
What’s Regulated and What’s Not?
The U.S. Environmental Protection Agency (EPA) currently regulates 11 disinfection by-products — including a handful of trihalomethanes (THM) and haloacetic acids (HAA). While these represent only a small fraction of all disinfection by-products, EPA aims to use their presence to indicate the presence of other disinfection by-products. “The general idea is if you control THMs and HAAs, you implicitly or by default control everything else as well,” says Korshin.
EPA also requires drinking water facilities to use techniques to reduce the concentration of organic materials before applying disinfectants, and regulates the quantity of disinfectants that systems use. These rules ultimately can help control levels of disinfection by-products in drinking water.
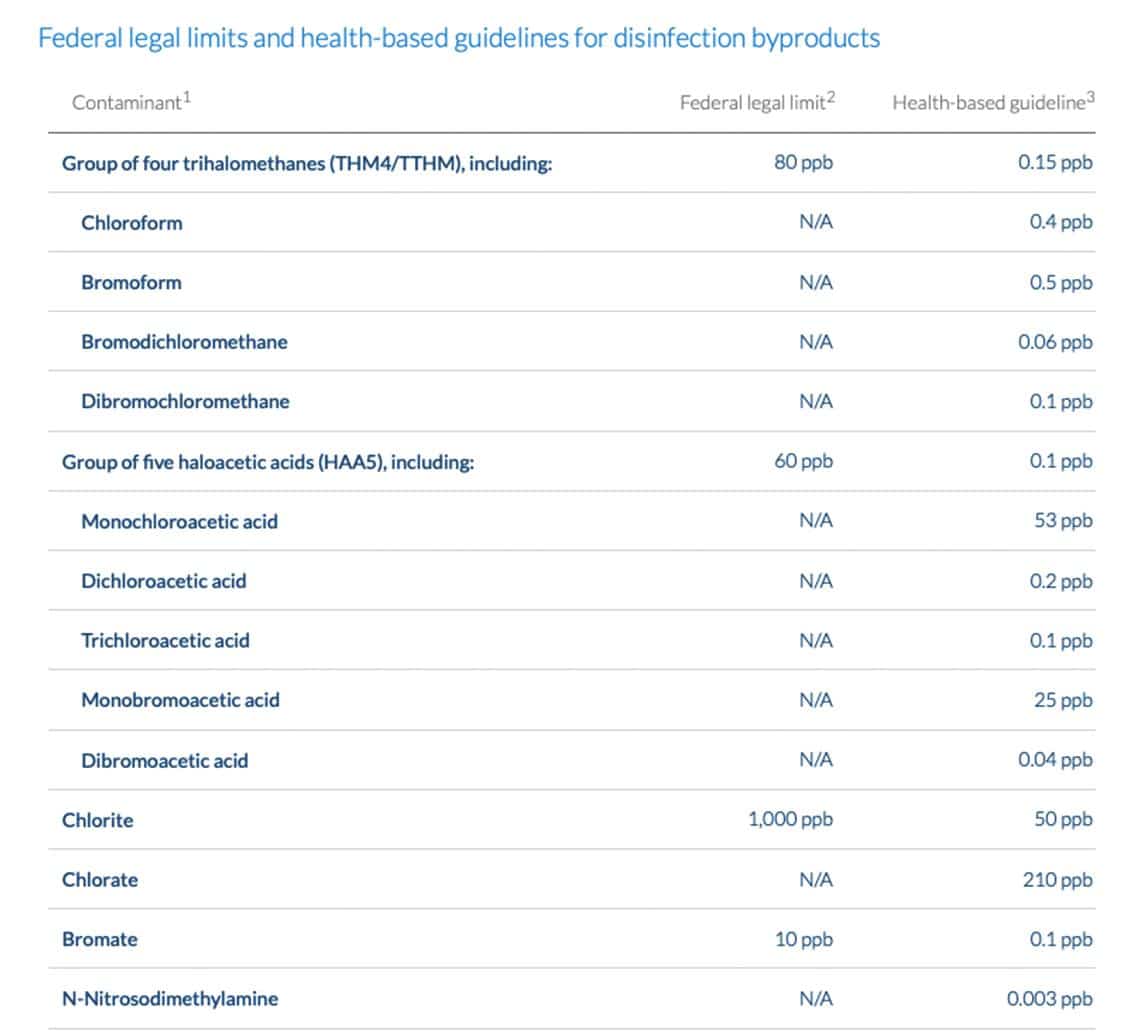
Click the image for an interactive version of this chart on the Environmental Working Group website.
Still, some scientists and advocates argue that current regulations do not go far enough to protect the public. Many question whether the government is regulating the right disinfection by-products, and if water systems are doing enough to reduce disinfection by-products. EPA is now seeking public input as it considers potential revisions to regulations, including the possibility of regulating additional by-products. The agency held a two-day public meeting in October 2020 and plans to hold additional public meetings throughout 2021.
When EPA set regulations on disinfection by-products between the 1970s and early 2000s, the agency, as well as the scientific community, was primarily focused on by-products of reactions between organics and chlorine — historically the most common drinking water disinfectant. But the science has become increasingly clear that these chlorinated chemicals represent a fraction of the by-product problem.
For example, bromide or iodide can get caught up in the reaction, too. This is common where seawater penetrates a drinking water source. By itself, bromide is innocuous, says Korshin. “But it is extremely [reactive] with organics,” he says. “As bromide levels increase with normal treatment, then concentrations of brominated disinfection by-products will increase quite rapidly.”
Emerging data indicate that brominated and iodinated by-products are potentially more harmful than the regulated by-products.
Almost half of the U.S. population lives within 50 miles of either the Atlantic or Pacific coasts, where saltwater intrusion can be a problem for drinking water supplies. “In the U.S., the rule of thumb is the closer to the sea, the more bromide you have,” says Korshin, noting there are also places where bromide naturally leaches out from the soil. Still, some coastal areas tend to be spared. For example, the city of Seattle’s water comes from the mountains, never making contact with seawater and tending to pick up minimal organic matter.
Hazardous disinfection by-products can also be an issue with desalination for drinking water. “As desalination practices become more economical, then the issue of controlling bromide becomes quite important,” adds Korshin.
Other Hot Spots
Coastal areas represent just one type of hot spot for disinfection by-products. Agricultural regions tend to send organic matter — such as fertilizer and animal waste — into waterways. Areas with warmer climates generally have higher levels of natural organic matter. And nearly any urban area can be prone to stormwater runoff or combined sewer overflows, which can contain rainwater as well as untreated human waste, industrial wastewater, hazardous materials and organic debris. These events are especially common along the East Coast, notes Sydney Evans, a science analyst with the nonprofit Environmental Working Group (EWG, a collaborator on this reporting project).
The only drinking water sources that might be altogether free of disinfection by-products, suggests Richardson, are private wells that are not treated with disinfectants. She used to drink water from her own well. “It was always cold, coming from great depth through clay and granite,” she says. “It was fabulous.”
Today, Richardson gets her water from a city system that uses chloramine.
Toxic Treadmill
Most community water systems in the U.S. use chlorine for disinfection in their treatment plant. Because disinfectants are needed to prevent bacteria growth as the water travels to the homes at the ends of the distribution lines, sometimes a second round of disinfection is also added in the pipes.
Here, systems usually opt for either chlorine or chloramine. “Chloramination is more long-lasting and does not form as many disinfection by-products through the system,” says Steve Via, director of federal relations at the American Water Works Association. “Some studies show that chloramination may be more protective against organisms that inhabit biofilms such as Legionella.”
If a drinking water facility fails to meet EPA regulations for disinfection by-products, one relatively easy and cheap modification is to add ammonia to the existing treatment, turning chlorine to chloramine. Many large community water systems in the U.S. now use chloramine. By doing so, according to Richardson, they have dropped levels of regulated disinfection by-products by up to as much as 90%.
However, there is one major drawback to this shift: the creation of potentially more harmful by-products. “It might push down on regulated disinfection by-products, but then other things pop up that are even more toxic,” says Richardson, whose research team discovered previously unknown disinfection by-products in chloraminated drinking water. One of those finds, iodoacetic acid, is the most DNA-damaging disinfection by-product known to date.
Prasse underscored the concern: “From a regulatory perspective, we could say we’re fine. But it’s a false sense of security.”
Rather than continuing on the toxic treadmill of replacing one potentially toxic chemical for another, a more effective solution may be to focus upstream in the treatment process — such as keeping organics out of the system in the first place. “That requires engineers, chemists, toxicologists and regulators to come together and figure something out,” says Prasse.
Alternative Approaches
When he moved to the U.S. from Germany, Prasse says he immediately noticed the bad taste of the water. “You can taste the chlorine here. That’s not the case in Germany,” he says.
In his home country, water systems use chlorine — if at all — at lower concentrations and at the very end of treatment. In the Netherlands, chlorine isn’t used at all as the risks are considered to outweigh the benefits, says Prasse. He notes the challenge in making a convincing connection between exposure to low concentrations of disinfection by-products and health effects, such as cancer, that can occur decades later. In contrast, exposure to a pathogen can make someone sick very quickly.
But many countries in Europe have not waited for proof and have taken a precautionary approach to reduce potential risk. The emphasis there is on alternative approaches for primary disinfection such as ozone or ultraviolet light. Reverse osmosis is among the “high-end” options, used to remove organic and inorganics from the water. While expensive, says Prasse, the method of forcing water through a semipermeable membrane is growing in popularity for systems that want to reuse wastewater for drinking water purposes.
Remucal notes that some treatment technologies may be good at removing a particular type of contaminant while being ineffective at removing another. “We need to think about the whole soup when we think about treatment,” she says. What’s more, Remucal explains, the mixture of contaminants may impact the body differently than any one chemical on its own.
Richardson’s preferred treatment method is filtering the water with granulated activated carbon, followed by a low dose of chlorine.
Granulated activated carbon is essentially the same stuff that’s in a household filter. (EWG recommends that consumers use a countertop carbon filter to reduce levels of disinfection by-products.) While such a filter “would remove disinfection by-products after they’re formed, in the plant they remove precursors before they form by-products,” explains Richardson. She coauthored a 2019 paper that concluded the treatment method is effective in reducing a wide range of regulated and unregulated disinfection by-products.

Greater Cincinnati Water Works installed a granulated activated carbon system in 1992, and is still one of relatively few full-scale plants that uses the technology. Courtesy of Greater Cincinnati Water Works.
Despite the technology and its benefits being known for decades, relatively few full-scale plants use granulated active carbon. They often cite its high cost, Richardson says. “They say that, but the city of Cincinnati [Ohio] has not gone bankrupt using it,” she says. “So, I’m not buying that argument anymore.”
Greater Cincinnati Water Works installed a granulated activated carbon system in 1992. On a video call in December, Jeff Swertfeger, the superintendent of Greater Cincinnati Water Works, poured grains of what looks like black sand out of a glass tube and into his hand. It was actually crushed coal that has been baked in a furnace. Under a microscope, each grain looks like a sponge, said Swertfeger. When water passes over the carbon grains, he explained, open tunnels and pores provide extensive surface area to absorb contaminants.
While the granulated activated carbon initially was installed to address chemical spills and other industrial contamination concerns in the Ohio River, Cincinnati’s main drinking water source, Swertfeger notes that the substance has turned out to “remove a lot of other stuff, too,” including PFAS and disinfection by-product precursors.
“We use about one-third the amount of chlorine as we did before. It smells and tastes a lot better,” he says. “The use of granulated activated carbon has resulted in lower disinfection by-products across the board.”
Richardson is optimistic about being able to reduce risks from disinfection by-products in the future. “If we’re smart, we can still kill those pathogens and lower our chemical disinfection by-product exposure at the same time,” she says.
Reposted with permission from Ensia.
- Toxic 'Forever Chemicals' Were Dropped Over Millions of Acres via ...
- Former Michigan Gov. Rick Snyder Among Officials to Be Charged ...
- 60 Million Americans Don’t Drink Their Tap Water – Here’s Why That’s a Public Health Problem
- California Public Health Crisis Looms With Water-System Failures
- Forever Chemicals Found in Home Fertilizers

 233k
233k  41k
41k  Subscribe
Subscribe 